New biomarker could flag tumors that are sensitive to common diabetes drug
May 19, 2020
GRAND RAPIDS, Mich. (May 19, 2020) — A newly identified biomarker could help scientists pinpoint which cancers are vulnerable to treatment with biguanides, a common class of medications used to control blood sugar in Type 2 diabetes.
Biguanides, particularly a medication called metformin, have long been of interest to cancer researchers because of their ability to target cellular metabolism, which fuels the growth and spread of malignant cells. To date, the success of biguanides as potential cancer therapeutics has been mixed, largely due to the difficulty in getting enough of the agent into cancer cells to be effective and the lack of a way to determine which cancers will respond to treatment.

“Cancers vary widely in how they react to different therapies — what works for one cancer type may not work for another — but regardless, they are all reliant on metabolism for energy production,” said Van Andel Institute Professor Russell Jones, Ph.D., the study’s senior author and leader of VAI’s Metabolic and Nutritional Programming group. “Our results establish two important things: First, they give us a way to objectively determine which types of cancer are sensitive to biguanide treatment and, second, they illuminate how and why some patients may respond better to biguanides than other patients.”
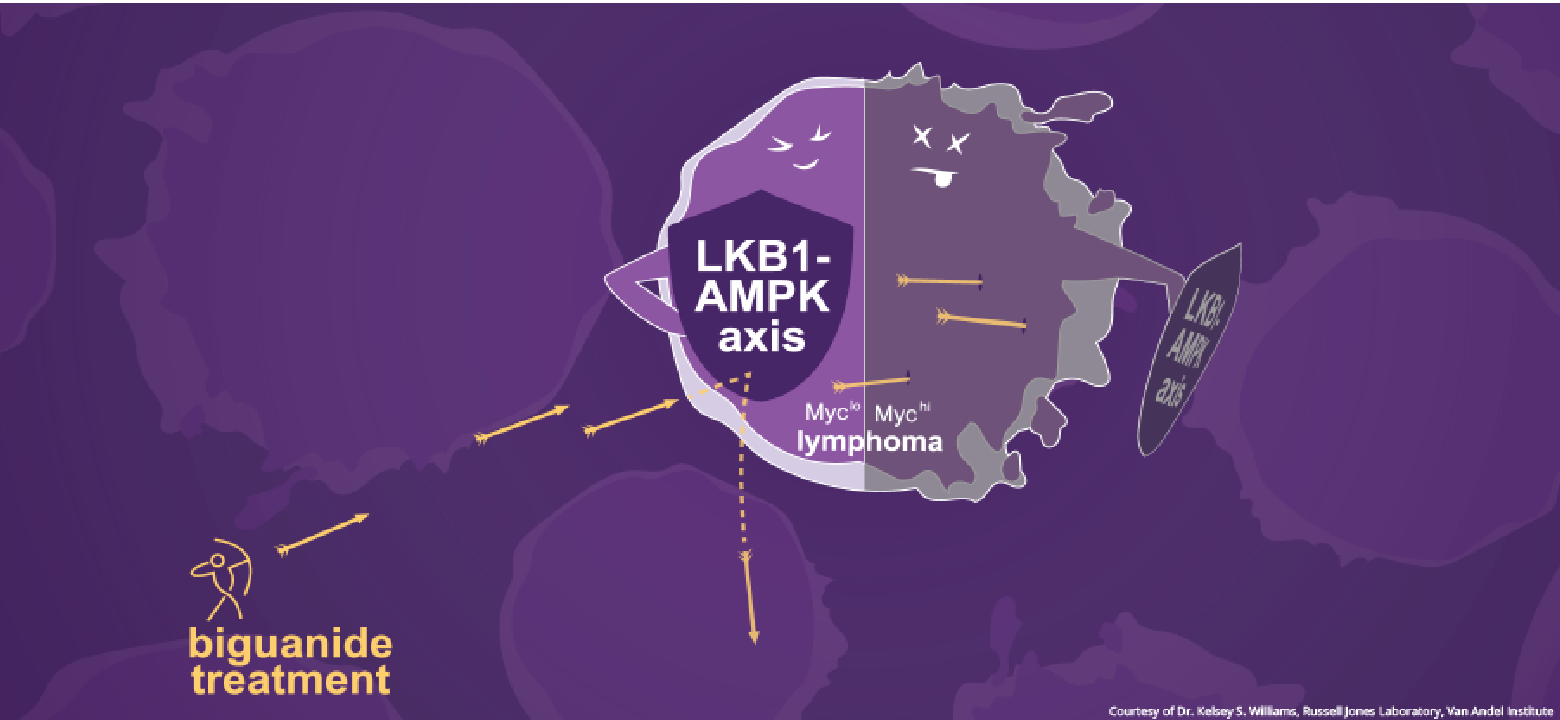
Published today in Cell Reports Medicine, the findings identify a microRNA regulated by the gene MYC as a biomarker for cancers that are sensitized to biguanide treatment. MYC is a well-known cancer-related gene whose activity is increased in as many as 70% of lymphomas. MYC works in part by turning down the activity of other genes that suppress tumor growth while heightening metabolic activity in cancer cells, a combination that allows the cells to flourish.
But there’s a trade-off. While MYC helps fuel cancer cells’ voracious appetites, it also turns off cells’ ability to respond to a stressful biological environment, limiting flexibility in their metabolism. Treatment with biguanides cut off this energy supply, causing stress that the cells cannot cope with and leading them to die. In Type 2 diabetes, biguanides are used to lower blood sugar, but in certain cancer cells, such as lymphomas with high MYC expression, the increased stress kills the cancer cells.
“Biguanides have great potential as cancer treatments, particularly for blood cancers,” Jones said. “Biomarkers such as what we have found here are vital tools for determining which cancers will respond to biguanides and which will not, which is important for patient care as well as designing more effective clinical trials.”
As part of the study, Jones and his colleagues also characterized an experimental biguanide called IM156, which is more potent than existing biguanides. IM156 was developed by ImmunoMet Therapeutics, a clinical-stage biotechnology company that develops anti-tumor and immunometabolism-based therapies. Jones serves as a member of ImmunoMet’s Board of Scientific Advisors.
In addition to Jones, authors include Said Izreig, Alexandra Gariepy, Ariel O. Donayo, Gaëlle Bridon, Daina Avizonis, Ph.D., and Thomas F. Duchaine, Ph.D., of Goodman Cancer Research Centre, McGill University; Irem Kaymak, Ph.D., Lisa M. DeCamp, Susan M. Kitchen-Goosen, Ryan D. Sheldon, Ph.D., and Kelsey S. Williams, Ph.D., of Van Andel Institute; Hannah R. Bridges, Ph.D., of University of Cambridge; Rob Laister, Ph.D., and Mark D. Minden, Ph.D., M.D., FRCPC, of Princess Margaret Cancer Centre, University of Toronto; Nathalie A. Johnson, Ph.D., and Michael N. Pollak, M.D., of Lady Davis Institute, McGill University; Marc S. Rudoltz, M.D., and Sanghee Yoo, Ph.D., of ImmunoMet Therapeutics. The Goodman Cancer Research Centre Metabolomics Core Facility and the Metabolomics and Bioenergetics Core at Van Andel Institute contributed to this work.
Research reported in this publication was supported by the Canadian Institutes of Health Research under grants MOP-142259 (Jones) and MOP-123352 (Duchaine); The Medical Research Council under grant MC_UU_0015/2 (Hirst); and funding from ImmunoMet Therapeutics. The Goodman Cancer Research Center Metabolomics Core Facility is supported by grants from the Canadian Foundation for Innovation, Canadian Institutes of Health Research and Terry Fox Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
###
ABOUT VAN ANDEL INSTITUTE
Van Andel Institute (VAI) is committed to improving the health and enhancing the lives of current and future generations through cutting edge biomedical research and innovative educational offerings. Established in Grand Rapids, Michigan, in 1996 by the Van Andel family, VAI is now home to more than 400 scientists, educators and support staff, who work with a growing number of national and international collaborators to foster discovery. The Institute’s scientists study the origins of cancer, Parkinson’s and other diseases and translate their findings into breakthrough prevention and treatment strategies. Our educators develop inquiry-based approaches for K-12 education to help students and teachers prepare the next generation of problem-solvers, while our Graduate School offers a rigorous, research-intensive Ph.D. program in molecular and cellular biology. Learn more at vai.org.
Media Contact
Beth Hinshaw Hall
Van Andel Institute
[email protected]
616-822-2064
Media assets available: Portrait of Dr. Russell Jones
